In this article we will discuss about:- 1. Definition of Ice Cream 2. History and Development of Ice Cream 3. Classification 4. Composition 5. Ingredients 6. Food and Nutritive Value 7. Role of the Constituents 8. Distribution 9. Soft Ice Cream (Softy) 10. Judging and Grading.
Contents:
- Definition of Ice Cream
- History and Development of Ice Cream
- Classification of Ice Cream
- Composition of Ice Cream
- Ingredients of Ice Cream
- Food and Nutritive Value of Ice Cream
- Role of the Constituents of Ice Cream
- Distribution of Ice Cream
- Soft Ice Cream (Softy)
- Judging and Grading of Ice Cream
1. Definition of Ice Cream:
Ice cream as an industry in India is of comparatively recent origin and may be said to have started in the nineteen-sixties. Today ice cream may be considered a luxury food item, although its popularity is increasing rapidly. The production of ice cream in India in 1966 was estimated to be about 0.7 per cent of the total milk production and 1.3 per cent of the milk used for manufacture of dairy products.
Ice cream may be defined as a frozen dairy product made by suitable blending and processing of cream and other milk products, together with sugar and flavour, with or without stabilizer or colour, and with the incorporation of air during the freezing process.
According to the PFA Rules (1976), ice cream is the frozen product obtained from cow or buffalo milk or a combination thereof or from cream, and or other milk products, with or without the addition of cane sugar, eggs, fruits, fruit juices, preserved fruits, nuts, chocolate, edible flavours and permitted food colours. It may contain permitted stabilizers and emulsifiers not exceeding 0.5 per cent by weight.
The mixture must be suitably heated before freezing. The product should contain not less than 10 per cent milk fat, 3.5 per cent protein and 36.0 per cent total solids. However, when any of the aforesaid preparations contain fruits or nuts or both, the content of milk fat may be proportionately reduced but may not be less than 8 per cent by weight. Starch may be added to a maximum extent of 5 per cent, with a declaration to that effect on the label.
2. History and Development of Ice Cream:
The early history of ice cream manufacture is very scanty; however, the product is definitely known to have originated in Europe. Water ices were made in southern Europe as early as the fifteenth century. The first printed record of ‘Cream ice’ appeared in The Experienced English House Keeper in 1769, more than two hundred years ago.
Since that time ice cream manufacture has continued to grow in popularity in England, though not nearly as rapidly as in the United States, where surveys indicate that ice cream forms a part of the daily diet and is the favourite American dessert.
Some of the factors contributing to the development of the ice cream industry in developed dairying countries are:
(i) The perfection of mechanical refrigeration and its application to the food industry;
(ii) Improved manufacturing methods and equipment such as homogenizers, overrun testers, continuous freezers, packaging machines, etc.;
(iii) More and better ingredients and growth in knowledge concerning ice cream manufacture, resulting in a better product;
(iv) Lower manufacturing costs through mass production;
(v) Extensive advertising of the product:
(vi) A realization of the high food value of ice cream;
(vii) Changing economic conditions, better wages, more purchasing power and a high standard of living among consumers;
(viii) improved storage facilities for ice cream at home.
3. Classification of Ice Cream:
No standard classification of ice cream has yet been adopted by the industry, even in developed countries.
However, some of the important frozen desserts can be classified as follows:
(i) Plain:
An ice cream in which the colour and flavouring ingredients together amount to less than 5 per cent of the volume of the unfrozen ice cream. Examples- Vanilla and Coffee ice creams.
(ii) Chocolate:
Ice cream flavoured with cocoa or chocolate.
(iii) Fruit:
Ice cream containing fruits, with or without additional fruit flavouring or colour. Fruits such as strawberry, apricot, pineapple, mango, banana, etc., may be fresh, frozen-packed, canned or preserved.
(iv) Nut:
Ice cream containing nuts, such as almonds, pistachio, walnuts, cashewnut, etc., with or without additional flavouring or colour.
(v) Milk Ices or Milk Lollies:
According to the PFA rules (1976), these refer to the frozen product obtained from milk, skim milk or milk products with or without the addition of cane sugar, eggs, fruits, fruit juices, nuts, chocolates, edible flavours, and permitted food colours. It may contain permitted stabilizers not exceeding 0.5 per cent of the product. The mixture should be suitably heat-treated before freezing. The product should contain no more than 2.0 per cent milk fat, not less than 3.5 per cent proteins and not less than 20.0 per cent total solids.
(vi) Ices:
Made of fruit juice, sugar and stabilizer, with or without additional fruit acid, colour, flavouring or water, and frozen to the consistency of ice cream. Usually contain 28 to 30 per cent sugar, 20 to 25 per cent overrun and no dairy products.
(vii) Sherbet:
Made of fruit juices, sugar, stabilizer, and milk products. It is similar to an ice except that milk, either whole, skim, condensed or powdered, or ice cream mix, are used in place of all or part of the water in an ice.
(viii) Fancy Moulded:
Moulded in fancy shapes and composed either of one colour and flavour of ice cream or a combination of colours and flavours, or especially decorated. Examples are- brick ice cream, cakes, cake roll, moulds representing fruits, etc.
(ix) Novelties:
A Novelty ice cream or frozen confection is an especially shaped and usually a low-priced package containing an individual serving whose main appeal consists in its shape, size, colour or convenience for eating.
(x) Soft Ice Cream (Softy):
Sold as drawn from the freezer without hardening.
4. Composition of Ice Cream:
The composition of ice cream is usually expressed as a percentage of its constituents, i.e., a percentage of milk fat, milk-solids-not-fat (serum solids), sugar, stabilizer, total solids, etc. Its composition varies in different localities and in different markets. The best ice cream composition for a manufacturer to produce is often difficult to establish.
After consideration of legal requirements, quality of product desired, raw materials available, plant procedures, trade demands, competition and cost, there is a choice of a product of minimum, average or high fat/solids composition. Some factories may choose to manufacture only one of these products, others two and still others all three, i.e., an economy-brand product, a good average composition product as a trade brand, or a deluxe high- quality product.
It may be inadvisable for a small manufacturer to produce more than one brand of ice cream. If only one composition is manufactured, it is extremely important that every effort be made to produce the best product possible.
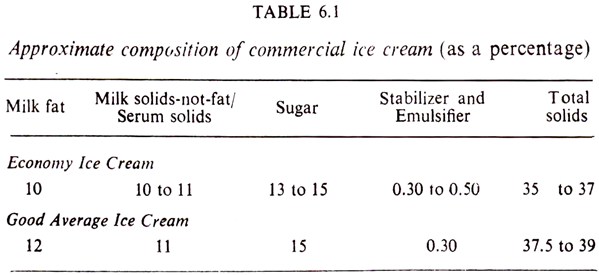
In ice cream, the percentage of milk fat varies more than any other constituent. As the fat content is increased, the milk-solids- not-fat must be decreased so as to avoid ‘sandiness’ (i.e. the crystallization of milk sugar or lactose in the finished ice cream). Table 6.1 gives the composition of economy and good average ice creams.
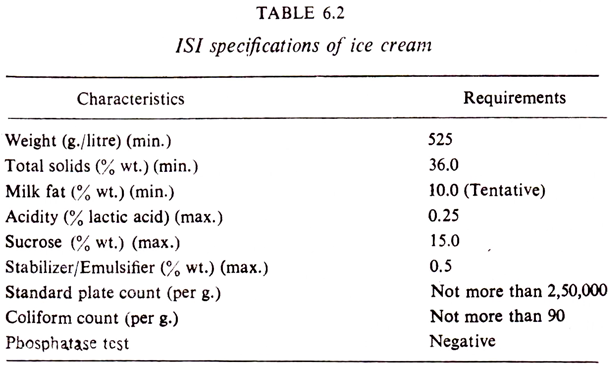
The ISI specifications for ice cream (IS: 2802, 1964) are given in Table 6.2.
Note:
A satisfactory composition produces an ice cream which has the desired combinations of cost, food value, flavour, body and texture, cooling effect, colour scheme, viscosity, whipping ability (i.e., overrun) and freezing point. What is needed is a balanced mix, in which the proportions of the constituents and ingredients is such as will produce a fine and satisfactory ice cream.
5. Ingredients of Ice Cream:
1. Dairy Products:
These constitute the basic materials for ice cream preparation.
2. Sweetening Agents:
Many kinds of sweeteners are used in ice cream. These include- cane and beet sugar, corn sweeteners, brown sugar, honey, invert sugar and sugar substitutes. Cane or beet sugars are most commonly used. One-fourth to one-third of cane or beet sugar may be replaced by corn sugar with good results.
The use of a combination or blend of sugars in either dry or liquid forms is a popular practice; sugar blends usually consist of 70 per cent sucrose and 30 per cent corn sweeteners. The different kinds of sugars do not produce an equal sweetening effect.
Apart from sweetening, sugars affect the properties of the mix and the finished product. Sugars depress the freezing point of the mix, produce a thinner mix with a slower whipping rate, and an ice cream with a smoother body and texture with faster melting qualities.
The different sweeteners commonly used are as follows:
(i) Sucrose:
Commonly known as granulated cane or beet sugar, this is the most widely accepted source of sugar throughout the world. It depresses the freezing point of ice cream.
(ii) Corn Sweeteners:
These may be- refined corn sugar (dextrose) —a dry crystalline product; dried corn syrup or corn syrup solids; and corn syrup—a liquid.
Refined corn Sugar—dextrose is 80 per cent as sweet as sucrose. It has a lower freezing point than sucrose and can therefore be used to about 25 per cent of the total desired sugar.
Dried corn Syrup Solids— contain dextrose and maltose together with dextrin. Usually added to the extent of not more than one-third of the total sweetener.
Corn syrup— contains variable amounts of dextrose and maltose.
(iii) Invert Sugar:
Mixture of different parts of glucose and fructose (resulting from hydrolysis of sucrose) and generally obtained in the form of syrup. Sweeter than sucrose. Depresses the freezing point and hence should be used to supply not more than one-fourth to one-third of the total sugar in the mix.
(iv) Saccharin:
It is an artificial sweetener. Sweetening effect up to 550 times that of sucrose. Used in ‘diabetic ice cream’. Causes problems of low total solids and low overrun. (Continued consumption of 0.3 g. per day is liable to impair digestion, because of its antiseptic properties and preservative action. Lately, it has been suggested that it might be a cause of cancer.)
Note:
Use of lactose as sweetener has the following problems- lactose is one-sixth as sweet as sucrose and is less soluble; moreover the crystals are hard and have sharp edges. When the water portion of the mix contains as much as 9 per cent lactose, the lactose may form crystals large enough to be discernible, and leave an undesirable ‘sandy’ taste in the mouth.
This property definitely limits the concentration of lactose in ice cream mix. Since the source of lactose is milk-solids-not-fat of dairy products and about 54 per cent of milk-solids-not-fat is lactose, the maximum concentration of lactose that can be safely used is directly related to the maximum concentration of milk-solids-not-fat.
3. Stabilizers:
These may be defined as substances which help to preserve emulsions. Although added in small amounts, they perform a very important role. Stabilizers are added in ice cream to produce smoothness in body and texture, retard or reduce ice crystal growth during storage, and provide uniformity in the product and resistance to melting. Stabilizers function through their ability to form gel structures in water or combine with water as water of hydration.
Among the stabilizers which are permitted and used in making ice cream are- gelatin, sodium alginate, carageenan, agar, carboxy methyl cellulose, pectin; guar gum, and other gums.
These are discussed below:
(i) Gelatin:
Of animal origin, it was one of the first of the commercial stabilizers, and is still used. Its advantage lies in its ability to form a gel in the mix during the ageing period as well as during the freezing process, and even after the frozen product is placed in the hardening room. Its peculiar gel structure and its great affinity for water prevent the formation of large ice crystals in ice cream, and contribute to the smoothness in texture and firmness in body of the frozen product.
The amount of gelatin to be used depends on several factors such as the source of gelatin, whether from calf, pork skins or bone material; its gel strength as measured by the Bloom Test; its viscosity value; the composition of the mix, etc.
In general, the amount to be used is approximately the amount required to produce a meltdown (in evenly melted ice cream) of about the same consistency as aged 40 per cent sweet cream.
This amount is usually between 0.25 and 0.5 per cent for a 250 Bloom gelatin. The ice cream mix stabilized with gelatin usually requires about 4 hours of ageing to develop complete stabilizing properties, while other stabilizing materials do not require an ageing period.
Note:
Several tests have been developed to determine gel strength and to serve as the guidelines to the amount to be used in the mix. Among these are the Bloom Test (by a Bloom Gelometer) for gel strength. Other factors being equal, the gelatin that carries the greatest gel strength per unit of cost is the one to select. Good quality gelatin should have a low bacteria) count and should be practically odourless and colourless.
(ii) Sodium Alginate:
Of vegetable origin; it is also sold under the trade name of ‘Dariloid’. The basic stabilizing principle, algin, is extracted from a giant ocean kelp (seaweed) growing on the shores of California and Japan. This product improves whipping ability and leaves a slightly cleaner flavour in the mouth. It dissolves properly only when added to the mix at about 68 to 71°C (155 to 160°F). A slightly smaller amount is needed to produce the same stabilizing effect as gelatin.
(iii) Carageenan:
This is extracted from Carageen (Irish Moss), a seaweed growing on the coast of Ireland, etc. It is used together with gum stabilizers.
(iv) Agar Agar:
This is a product extracted from red algae (seaweed) growing on the Pacific Coast. More useful for sherbets and ices.
(v) CMC (Carboxy Methyl Cellulose):
This has a high water-holding capacity and can be easily dissolved in the mix. Also acts as an emulsifier. Slightly less of it is to be used than gelatin. It does not form as firm a gel as gelatin and some of the vegetable stabilizes, but seems to be good for use in ice cream.
(vi) Pectin:
This is a carbohydrate obtained mainly from citrus fruits. Not satisfactory as a stabilizer for ice cream, although suitable for sherbets and ices.
(vii) Guar Gum:
This is a carbohydrate obtained from Indian legume. Often used in combination with carrageenan. It is readily soluble in cold solutions and used as stabilizer for mixes to be pasteurized by HTST or continuous pasteurization methods.
(viii) Other Gums (such as Tragacanth, Arabic, Karaya, or India Gums):
These are exudates from incisions made in the bark of certain trees and plants growing usually in tropical countries. More useful for sherbets and ices.
Note:
(i) The selection of a suitable stabilizer for use in the mix is dependent on several factors, such as- adaptability to the particular need of the plant; personal preference; availability; cost; freedom from toxicity; effect of stabilizer on the properties of the mix; whether or not an ageing period is necessary; kind of product being manufactured and method of processing adopted in the plant; effect on flavour and body and texture characteristics; and effect on melting and storage properties of the ice cream.
(ii) The amount of stabilizer used depends on the kind of stabilizer and the quality necessary to produce the desired stabilizing effect in the product being manufactured. There are four common ways of determining the amount of stabilizer to be used in the ice cream mix- the fat content of the mix; the total solids content of the mix; the kind of freezer used; and the use of a constant amount which may range from 0.15 to 0.50 per cent.
(iii) The method of incorporating stabilizers (and emuisifiers) in the ice cream mix is as follows- Mix with 4 to 5 parts of sugar and add before or during the heating process, disperse by gently adding to the mix without special handling, suspend in cold water and add them to the mix when mix temperature is 71°C (160°F) in the case of sodium alginate, or use a hopper and pump or some other special arrangement.
When the batch method of pasteurization is employed, the stabilizer may be added to the cold or hot mix. This method gives greater latitude for the selection of a stabilizer. In the HTST pasteurization process, the stabilizer must be added to the cold mix ingredients and should disperse readily at a low temperature; the algin, guar and CMC type of products are commonly used in this processing method.
4. Emulsifiers:
These may be defined as substances which help to form emulsions. The value of emulsifying agents in the manufacture of ice cream lies mainly in the improved whipping quality of the mix, the production of a drier ice cream with a smoother body and texture, in their superior drawing qualities at the freezer, and the possibility of maintaining more precise control over the various manufacturing processes.
There are two kinds of emulsifiers used in the manufacture of ice cream, viz., mono- and di-glycerides derived by the chemical reaction of naturally occurring glycerides and polyoxyethylene derivatives of hexahydric alcohols, glycol and glycol esters.
The mono-glycerides improve fat dispersion and whipping ability and have a moderate effect on stiffness and the melting rate. The poly-derivatives are effective in producing dryness, stiffness and increasing the melting time.
Emulsifiers are available in liquid, semi-solid and powder forms and may include glycerides, lecithin and fatty acid esters. In general, emulsifiers have little effect on the acidity, pH or viscosity of the ice cream mix. A significant reduction in whipping time is encountered when any emulsifier is used.
The use of emulsifiers decreases the melting rate in the finished ice cream. Emulsifiers seem to produce somewhat smaller ice crystals which are more evenly distributed, and smaller air cells that result in a smoother ice cream. Several factors may affect the action of an emulsifier. Among these are- ingredients of the mix; procedure of processing, freezing and hardening; and the amount of emulsifier used.
Some disadvantages in the use of emulsifiers are- homogenization of the mix is essential in order to obtain good results and they seem to favour the development of a ‘shrinkage’ defect. Excessive use of emulsifiers may cause a short body and poor texture, slow melting, and a curdy meltdown in the finished ice cream.
Note:
Excellent ice cream, and considerable amounts of it, are made without adding stabilizer or emulsifier. Since milk and milk products contain natural stabilizing and emulsifying materials, viz., milk protein, fat, lecithin, phosphates and citrates, mixes of a certain composition and processing treatment may be stabilized by the effect of these natural materials.
Further, egg yolk products are high in lecithin and have long been used in ice cream. These products produce results similar to, but not as pronounced as, those caused by commercial stabilizers and emulsifiers.
5. Flavours:
The two most popular flavours, viz., vanilla and chocolate, have been discussed in detail below.
6. Colours:
Only harmless, edible, permitted food colours should be used. Generally, colours are matched with the flavours added.
7. Egg Solids:
Frozen and powdered egg yolks are used by many ice cream manufacturers. Usually not more than 0.5 per cent of egg yolk solids are added to the mix. Egg yolk solids improve the whipping quality of the mix. They are especially desirable in mixes in which butter or butteroil constitute the main source of fat.
8. Fruits and Nuts:
The fruits in ice cream may be fresh, frozen or canned. Fresh fruits must be considered the best source of flavour when available at low prices; they should be thoroughly washed and peeled or hulled before use. Fruits may be used whole, sliced or crushed.
It is better to mix fruits with sugar in the ratio of 2-7 kg. Fruit to 1 kg. Sugar and hold them at about 5°C (40°F) for 12-24 hours before using @ 15-20 per cent of the mix. In India, pieces of chopped mango, banana, etc. are added towards the end of the freezing process @ 7-8 per cent of the mix. Care should be taken to exclude inedible and fibrous parts.
Nuts should be sound, clean, and free from rancid flavours. Considerable care should be taken to prepare them for the ice cream mix so that there are no foreign materials. To make them safe, nuts are dry-heated to pasteurizing temperatures or above; they may also be fried in oil or dipped in a boiling, slightly salted, sugar solution for a few seconds. All nuts should be chopped into very small pieces before they are added to the mix at 1-3 per cent.
6. Food and Nutritive Value of Ice Cream:
This depends not only on the composition of the ice cream but also upon the food and nutritive value of the products from which it is made. For instance, ice cream contains two to three times as much fat and slightly more protein than does milk. In addition, it may contain other food products such as fruits, nuts, eggs and sugar which enhance its food value. However, like milk, it lacks iron, vitamin C and some of the trace minerals.
Among milk products, ice cream is also a rich source of calcium, phosphorus and other minerals of vital importance in building good bones and teeth. Being rich in lactose, ice cream favours greater assimilation of the calcium content in the diet. The protein content of ice cream also rates high, both in quantity and quality.
The proteins are largely derived from milk, a small amount from stabilizer (gelatin) and from eggs when they are used in the mix. The milk and egg proteins are complete; that is, they contain all the amino-acids essential to animal life and are especially important sources of tryptophane and lysine which are lacking in many plant proteins. Ice cream provides these valuable proteins in a very palate table form. In fact, ice cream (without eggs and gelatin) is the most palatable source of milk proteins to vegetarians.
Ice cream is an excellent source of food energy. Having twice to three times the fat content of milk, and more than half its total solids being sugar (sucrose and lactose), the energy value of ice cream is very high. It is, therefore, a very desirable food item for growing children and persons who need to put on weight.
Like milk, ice cream is a rich source of many essential vitamins, without which normal health and growth cannot be maintained. Thus it is an excellent source of vitamin A, a good source of vitamins B (Thiamine) and G (Riboflavin), and a fairly good source of Niacin, vitamin E, and in fruit ice cream, of vitamin C. The digestibility and palatability of ice cream is also very high.
7. Role of the Constituents in Ice Cream:
(a) Milk Fat:
This is high in food value, but expensive. It enriches and mellows the ice cream, giving it a full, rich, creamy flavour. If the milk fat is even slightly off-flavoured, the defect will be noticeable. The fat also contributes to the body and melting resistance of ice cream while producing a smoothness of texture. Fat gives stability to the ice cream but impairs whipping ability.
(b) Milk-Solids-Not-Fat (MSNF):
Also known as serum solids, they consist of milk proteins, milk sugar and mineral matter. They are high in food value and also inexpensive. They add very little to the smell, but improve its body and texture. However, milk sugar adds to the sweet taste. The milk proteins help to make ice cream more compact and smooth. Milk-solids not-fat should be added in as large a quantity as possible without risking the danger of sandiness.
(c) Sugar:
The main function of sugar is to increase the acceptability of ice cream. The desired sweetening effect is only produced by sucrose. Sugars are usually the cheapest source of total solids in the mix.
(d) Stabilizers:
These are used to prevent the formation of objectionably large ice crystals in ice cream, especially during storage. Since they arc added in very small quantities, they have a negligible influence on food value and flavour.
(e) Emulsifiers:
These are used mainly to improve upon and provide a uniform whipping quality to the mixture, and to produce a drier ice cream with smoother body and texture.
(f) Flavour and Colour:
Flavour increases the acceptability of ice cream, and colour its aesthetic appeal.
(g) Advantages and Limitations of Ice Cream Constituents:
The advantages and limitations of the various ice cream constituents have been summarized by Sommer as follows-
I. Milk Fat:
Advantages:
(i) Enriches the flavour; (ii) produces a characteristic smooth texture; (iii) helps give body to the ice cream.
Limitations:
(i) Cost; (ii) fat slightly hinders, rather than improves, whipping; (iii) high fat content may limit the amount of ice cream consumed; (iv) high calorific value.
II. Milk-Solids-Not-Fat:
Advantages:
(i) Improve the texture; (ii) help to give body; (iii) a higher overrun without snowy or flaky texture; (iv) a comparatively cheap source of solids.
Limitations:
(i) A high percentage causes ‘sandiness’; (ii) the ‘condensed milk’ flavour may be objectionable; (iii) may cause salty or cooked flavour.
III. Sugar:
Advantages:
(i) Is usually the cheapest source of solids; (ii) improves the texture; (iii) enhances the flavour.
Limitations:
(i) Excessively sweet; (ii) lowers whipping ability; (iii) requires a lower temperature for proper hardening.
IV. Stabilizer:
Advantages:
(i) Very effective in smoothening the texture; (ii) very effective in giving body to the product. Limitation, (i) Excess body and melting resistance.
V. Emulsifier:
Advantages:
(i) Improves whipping quality of mixture; (ii) gives smoother body and texture; (iii) reduces whipping time.
Limitations:
(i) Homogenization of milk is essential; (ii) tends to favour ‘shrinkage’ defect; (iii) excess body and melting resistance.
VI. Total Solids:
Advantages:
(i) Smoother texture; (ii) better body; (iii) more nutritious; (iv) ice cream not as cold.
Limitations:
(i) Heavy, soggy or pasty body; (ii) cooling effect insufficiently high.
VII. Flavour:
Advantage:
(i) Increases acceptability.
Limitations:
(i) Harsh flavour less desirable; (ii) intense flavours provide immediate satisfaction.
VIII. Colour:
Advantages:
(i) Improves appearance; (ii) aids in identifying flavours.
Limitations:
(i) Intense and ‘unnatural’ colours reduce consumer acceptability.
8. Distribution of Ice Cream:
When ice cream is marketed, the manufacturer usually transports it to the retailer under refrigeration at the same temperature as is maintained in the retailer’s cabinet.
The various means of refrigerating the distribution vehicle are:
(a) ‘Dry Ice’ Refrigeration:
Dry ice is solid carbon dioxide with a freezing point of — 78°C (— 109°F); it is used extensively for package deliveries, it is cut into pieces of an appropriate size, which are wrapped in paper to delay rapid evaporation, and then placed around the package of the ice cream inside an insulated packer or in a single service type packer. The latter is usually a cardboard box insulated with corrugated cardboard and is used especially for the carry-out package. This system is popular for retailing ice-cream in pushcarts in large Indian cities.
Advantages:
(i) It is neither moist nor messy;
(ii) The package is neat in appearance;
(iii) It does not waterlog the insulation, and
(iv) It is very light.
Disadvantages:
(i) At present, it is expensive and availability is limited;
(ii) Loss during handling and storage (up to 10 to 15%);
(iii) Danger of burns to ‘handler’ and
(iv) Greater opportunity for heat-shock, which may injure the texture of the ice cream.
(b) Refrigerated Truck:
This is the most commonly used means of transportation for ice cream in developed countries. The truck is refrigerated overnight, and loaded with hardened ice cream the following morning.
(c) Frozen Brine:
Jacketed metal containers, known as Eutectic pads, which contain calcium chloride (brine) solution of specific gravity of about I.I, are refrigerated in a similar brine tank whose specific gravity is about 1.26, at a temperature of —32°C ( —25°F). The containers attain a temperature of — 21°C (—5°F) and are then placed around the packaged ice cream. This arrangement is used by a few ice cream factories for air-lifting ice cream in this country.
9. Soft Ice Cream (Softy):
There is a marked demand for the form of ice cream which has generally come to be known as ‘softy’. This term has been applied largely because this product is marketed in a soft condition and is ready for consumption shortly after it is drawn from the freezer. The problems involved in the preparation of softy ice creams are somewhat different from those encountered in the manufacture of regular ice cream.
The composition of soft ice cream has been given in Table 6.7.
Note:
Soft ice cream is usually drawn from the freezer at around — 8 to —7°C (18 to 20°F). The overrun may be in the range of 30 to 50 per cent.
10. Judging and Grading of Ice Cream:
Score Card:
This is given in Table 6.8.
Procedure of Examination:
(a) Sampling:
Taken at random for ice cream cups but at a later stage for bigger lots.
(b) Tempering Ice Cream:
Generally, temperatures from — 15°C to — 12°C (5°F to 10°F) are satisfactory. This is best done by taking the ice cream out of the hardening room and placing it in a dispensing cabinet several hours prior to judging.
(c) Sequence of Observations:
Since the condition of ice cream changes rapidly when exposed to room temperature, one should be alert and observant during sampling, so as not to miss any of its true characteristics, particularly of body and texture.
Proceed in the following order:
(i) Examine Container:
Note the type and condition of container and presence of any package defects.
(ii) Note Colour of Ice Cream:
Observe the colour of ice cream, its intensity and uniformity, and whether the colour matches its flavour.
(iii) Sample the Ice Cream:
While using the dipper for sampling, note the following: the manner in which the product cuts, the evenness of cutting, the resistance offered during cutting, the presence of ice particles, whether the ice cream is heavy or soggy, light or fluffy, etc. Set aside a sample in a petri plate/dish for observing the melting characteristics.
(iv) Begin Judging:
After obtaining the sample, take a small spoonful or bite of the frozen product, and taste. Quickly manipulate the sample between teeth and tongue and note the body and texture and flavour characteristics. (Expect delayed taste reaction.)
Note:
Rinse mouth with saline water frequently.
(v) Note the Melting Qualities:
Observe whether the melted liquid is creamy, curdled, foamy or watery.
(vi) Determine the bacterial count.
Requirements of High Grade Ice Cream:
An ideal ice cream should be packaged in an attractive container, possess a typically pleasant and desirable flavour, have a close, smooth and uniform body and texture, have desirable melting properties, possess an uniform natural colour, and have a low bacterial count.